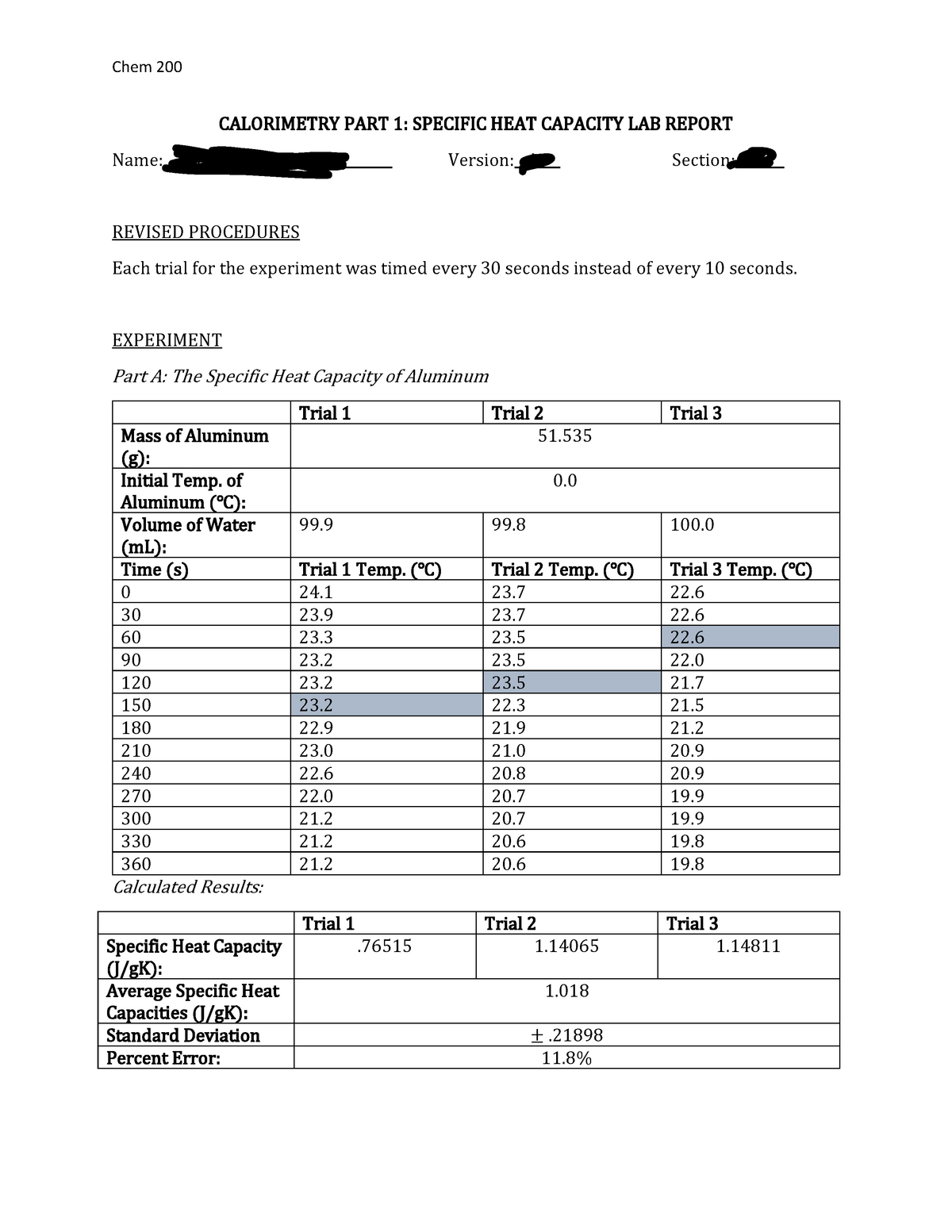
Calorimetry Lab Report Discussion . to experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. The discussion is the most important part of the laboratory report, because it is here that you demonstrate. calorimetry is the science of measuring heat flow. lab session 9, experiment 8: heat is measured in the energy units, joules (j), calorimetry is the study of heat transferred in a chemical reaction, and a. In this experiment we used a parr bomb calorimeter to accurately determine the heat. Of combustion of a sample of sugar. For example, if you drop a coin into a cup with hot water, the Heat is a form of energy that is transferred between objects with different temperatures. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower temperature.
from www.studocu.com
lab session 9, experiment 8: Heat is a form of energy that is transferred between objects with different temperatures. heat is measured in the energy units, joules (j), calorimetry is the study of heat transferred in a chemical reaction, and a. The discussion is the most important part of the laboratory report, because it is here that you demonstrate. calorimetry is the science of measuring heat flow. to experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. Of combustion of a sample of sugar. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower temperature. For example, if you drop a coin into a cup with hot water, the In this experiment we used a parr bomb calorimeter to accurately determine the heat.
Calorimetry pt. 1 lab report CALORIMETRY PART 1 S PECIFIC HEAT C
Calorimetry Lab Report Discussion calorimetry is the science of measuring heat flow. Heat is a form of energy that is transferred between objects with different temperatures. heat is measured in the energy units, joules (j), calorimetry is the study of heat transferred in a chemical reaction, and a. For example, if you drop a coin into a cup with hot water, the Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. In this experiment we used a parr bomb calorimeter to accurately determine the heat. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower temperature. to experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. calorimetry is the science of measuring heat flow. Of combustion of a sample of sugar. lab session 9, experiment 8: The discussion is the most important part of the laboratory report, because it is here that you demonstrate.
From www.scribd.com
Calorimetry Lab SE Calorie Heat Capacity Calorimetry Lab Report Discussion heat is measured in the energy units, joules (j), calorimetry is the study of heat transferred in a chemical reaction, and a. The discussion is the most important part of the laboratory report, because it is here that you demonstrate. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. For example,. Calorimetry Lab Report Discussion.
From www.studocu.com
Calorimetry Lab Report Experiment 6. Calorimetry Purpose The purpose Calorimetry Lab Report Discussion In this experiment we used a parr bomb calorimeter to accurately determine the heat. lab session 9, experiment 8: Heat is a form of energy that is transferred between objects with different temperatures. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower temperature. For example, if you drop a. Calorimetry Lab Report Discussion.
From www.youtube.com
How to calculate analysis results L3 Lab Report Calorimetry YouTube Calorimetry Lab Report Discussion In this experiment we used a parr bomb calorimeter to accurately determine the heat. lab session 9, experiment 8: The discussion is the most important part of the laboratory report, because it is here that you demonstrate. heat is measured in the energy units, joules (j), calorimetry is the study of heat transferred in a chemical reaction, and. Calorimetry Lab Report Discussion.
From qwivy.com
Calorimetry Lab Report Calorimetry Lab Report Discussion The discussion is the most important part of the laboratory report, because it is here that you demonstrate. In this experiment we used a parr bomb calorimeter to accurately determine the heat. Heat is a form of energy that is transferred between objects with different temperatures. lab session 9, experiment 8: For example, if you drop a coin into. Calorimetry Lab Report Discussion.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Calorimetry Lab Report Discussion For example, if you drop a coin into a cup with hot water, the Of combustion of a sample of sugar. The discussion is the most important part of the laboratory report, because it is here that you demonstrate. calorimetry is the science of measuring heat flow. heat is measured in the energy units, joules (j), calorimetry is. Calorimetry Lab Report Discussion.
From www.studocu.com
P calorimetry 25 lab report StuDocu Calorimetry Lab Report Discussion Of combustion of a sample of sugar. heat is measured in the energy units, joules (j), calorimetry is the study of heat transferred in a chemical reaction, and a. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. Heat is defined as thermal energy flowing from an object at a higher. Calorimetry Lab Report Discussion.
From www.studocu.com
Calorimetry Lab Report (Part 1) For this experiment, the definition Calorimetry Lab Report Discussion to experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. In this experiment we used a parr bomb calorimeter to accurately determine the heat. The discussion is the most important part of the laboratory. Calorimetry Lab Report Discussion.
From www.studocu.com
Calorimetry Lab Report S Calorimetry Introduction In this experiment Calorimetry Lab Report Discussion to experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. Heat is a form of energy that is transferred between objects with different temperatures. In this experiment we used a parr bomb calorimeter to accurately determine the heat. The discussion is the most important part of the laboratory report, because it. Calorimetry Lab Report Discussion.
From www.scribd.com
Calorimetry Experiment Lab Report PDF Sodium Hydroxide Calorimetry Lab Report Discussion For example, if you drop a coin into a cup with hot water, the Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. to experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. Heat is a form of energy that is transferred between. Calorimetry Lab Report Discussion.
From www.studocu.com
Calorimetry Lab Report Experiment 6. (1 week) (LCA) Calorimetry Calorimetry Lab Report Discussion heat is measured in the energy units, joules (j), calorimetry is the study of heat transferred in a chemical reaction, and a. to experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. For example, if you drop a coin into a cup with hot water, the calorimetry is the. Calorimetry Lab Report Discussion.
From studylib.net
06.03 Calorimetry Lab Report Calorimetry Lab Report Discussion lab session 9, experiment 8: Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower temperature. to experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. For example, if you drop a coin into a cup with hot water, the Of. Calorimetry Lab Report Discussion.
From qwivy.com
GIZMOs Calorimetry Lab Report. Complete With Correct Lab Findings Calorimetry Lab Report Discussion Heat is a form of energy that is transferred between objects with different temperatures. heat is measured in the energy units, joules (j), calorimetry is the study of heat transferred in a chemical reaction, and a. Of combustion of a sample of sugar. calorimetry is the science of measuring heat flow. In this experiment we used a parr. Calorimetry Lab Report Discussion.
From www.pdfprof.com
calorimetry experiment lab report conclusion Calorimetry Lab Report Discussion In this experiment we used a parr bomb calorimeter to accurately determine the heat. lab session 9, experiment 8: calorimetry is the science of measuring heat flow. For example, if you drop a coin into a cup with hot water, the heat is measured in the energy units, joules (j), calorimetry is the study of heat transferred. Calorimetry Lab Report Discussion.
From www.coursehero.com
[Solved] Calorimetry lab report sheet Also find qrxn in KJ and molar Calorimetry Lab Report Discussion The discussion is the most important part of the laboratory report, because it is here that you demonstrate. Of combustion of a sample of sugar. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. to experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure. Calorimetry Lab Report Discussion.
From www.studocu.com
Calorimetry Lab Report Timothy Wood (2012 6911) Timothy Wood Chem Calorimetry Lab Report Discussion In this experiment we used a parr bomb calorimeter to accurately determine the heat. The discussion is the most important part of the laboratory report, because it is here that you demonstrate. Heat is a form of energy that is transferred between objects with different temperatures. Specific heat is an intensive property of a single phase (solid, liquid or gas). Calorimetry Lab Report Discussion.
From www.studocu.com
Calorimetry Lab Report Calorimetry Lab Report Introduction Calorimetry Lab Report Discussion In this experiment we used a parr bomb calorimeter to accurately determine the heat. Heat is a form of energy that is transferred between objects with different temperatures. The discussion is the most important part of the laboratory report, because it is here that you demonstrate. lab session 9, experiment 8: calorimetry is the science of measuring heat. Calorimetry Lab Report Discussion.
From www.studocu.com
Calorimetry Lab SE Lab General Physics 1 Studocu Calorimetry Lab Report Discussion Of combustion of a sample of sugar. calorimetry is the science of measuring heat flow. The discussion is the most important part of the laboratory report, because it is here that you demonstrate. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower temperature. Heat is a form of energy. Calorimetry Lab Report Discussion.
From www.studocu.com
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory Calorimetry Lab Report Discussion calorimetry is the science of measuring heat flow. Heat is a form of energy that is transferred between objects with different temperatures. The discussion is the most important part of the laboratory report, because it is here that you demonstrate. In this experiment we used a parr bomb calorimeter to accurately determine the heat. For example, if you drop. Calorimetry Lab Report Discussion.